R+D+I: Kliniske Studier ved Instituto Bernabeu
A correct medical treatment must combine, together with dedicated care, the personalization of the process and in some cases, the application of R + D + I. At Instituto Bernabeu we are constantly carry out different lines of research to which, if they wish, our patients can join.

FINN ETTER TYPE EKSAMEN:
DET MEST RELEVANTE
A phase II, randomised, double-blind, parallel-group, placebo-controlled, double-blind test to evaluate the ongoing pregnancy rate with OXO-001 (200 mg, 300 mg) or placebo at 10 weeks after transfer of a single fresh blastocyst resulting from IVF/ICSI with donated eggs.
Objective: The primary objective is to investigate whether the trial drug will help improve the pregnancy rate in patients undergoing egg donation treatment.
Current status: Completed.
Location: Instituto Bernabeu Alicante, Instituto Bernabeu Madrid
Project responsibles: Dr Andrea Bernabeu, Dr Ana Fuentes, Dr Ruth Romero
More information: For more information, please contact the following email address: research@institutobernabeu.com
For more details on the study, please click on the following link.
Aim: This is a prospective, randomised study to identify whether there’s a relationship between the method of oocyte maturation and the number of oocytes obtained in a selected population with indicators of low response.
Current situation: Completed.
Location: Instituto Bernabeu Alicante, Instituto Bernabeu Madrid, Instituto Bernabeu Albacete, Instituto Bernabeu Palma de Mallorca
Coordinated by: Dr Jordi Suñol, Dr Andrea Bernabeu, Dr Cristina Gavilán, Dr Lydia Luque, Dr Ruth Romero
Morphokinetics and ploidy in human blastocysts
Investigation subject: the relationship that may exist between different morphokinetic events and the embryos’ chromosomal status.
Objective: to establish which morphokinetic parameters are related to embryonic aneuploidy in order to improve the selection criteria and transfer the embryo with the greatest implantation capacity to the maternal uterus.
Current situation: Completed.
Location: Instituto Bernabeu Alicante.
Coordinated by: Dr Jorge Ten and Marta Arrasate (student at the Master of Reproduction Medicine)
Investigation subject: a retrospective evaluation On what type of endometrial medical preparation (natural or artificial) provides the best clinical results for patients undergoing cryotransfer has been made.
Objective: to learn what type of endometrial preparation (natural or artificial) is the most appropriate and which one gives best results, depending on some important parameters such as the oocyte origin (own or donated) and the performance of pre-implantation genetic diagnosis for the detection ofaneuploidy.
Current situation: Completed.
Location: multicentre project of all Instituto Bernabeu clinics.
Coordinated by: Dr Jorge Ten, Jaime Guerrero and Esther Abellán (student at Master in Reproductive Medicine).
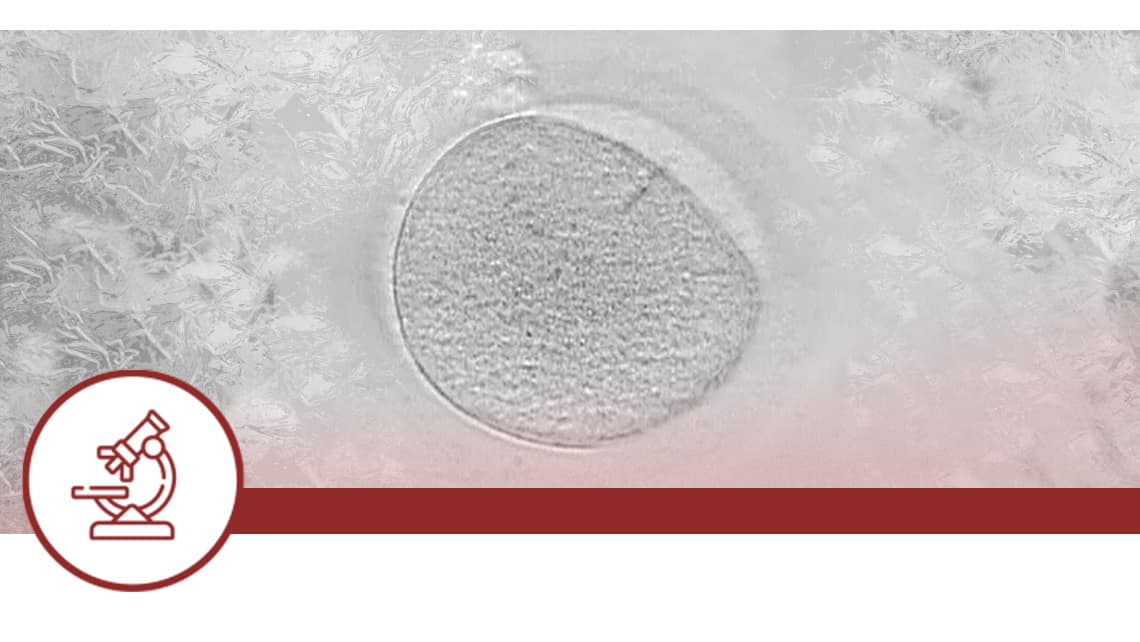
Effect of pre-vitrification embryo collapse on clinical outcomes after cryotransfer
Investigation subject: blastocysts collapse by applying a laser pulse improves embryo survival outcomes after vitrification and thawing and could also increase clinical outcomes.
Objective: to test whether laser-mediated blastocyst collapse has a beneficial effect on clinical outcomes in patients undergoing cryotransfer.
Current situation: study completed. Pending completion of clinical results up to live birth.
Location: multicentre project of all the Instituto Bernabeu clinics.
Coordinated by: Dr Jorge Ten and Alba Bellón (student at Master in Reproductive Medicine).
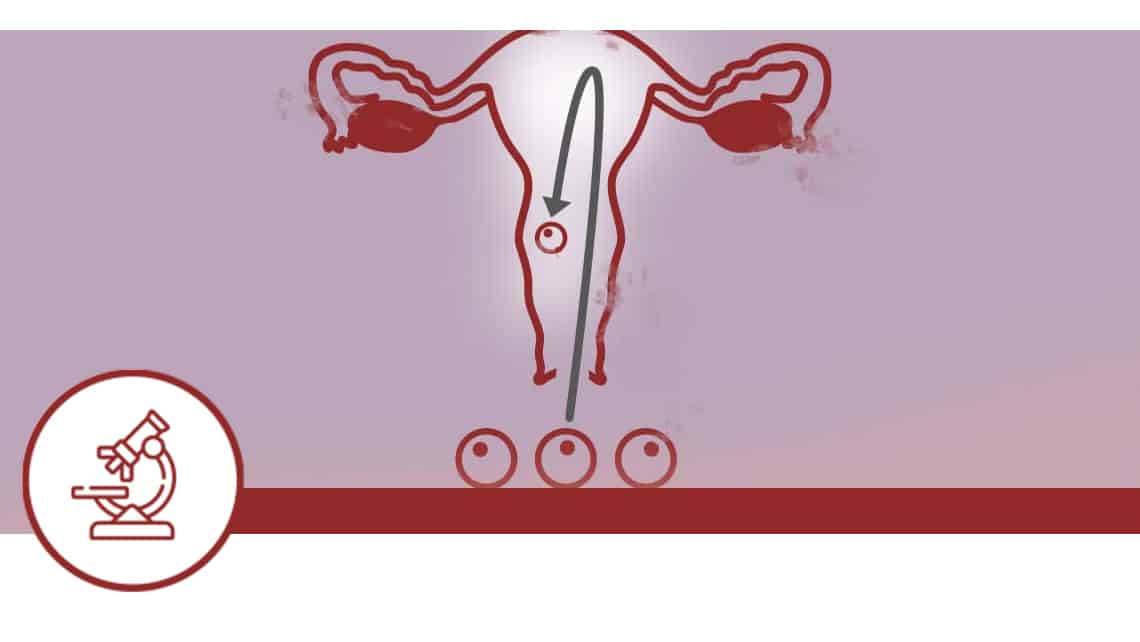
Double Donation Pregnancy Guarantee Evaluation Project
Investigation subject: Recurrent implantation failure represents a major cause of infertility difficult to explain, defined as the failure to establish a viable pregnancy after successive IVF treatments. It’s estimated that between 10% and 15% of women undergoing assisted reproduction treatments experience this type of problem. There’re many possible causes, including uterine, immunological, genetic and other factors. The woman, the male and the embryo are the three main actors in this situation, with the embryonic factor probably being the one most frequent behind these cases.
Objective: The main objective is to evaluate the prevalence of patients with implantation failure casuistry based on an ideal population, within a double gamete donation programme, thus mitigating the possible biases associated with the embryo factor.
Current situation: Basadate
Location: Instituto Bernabeu. Alicante.
Coordinated by: J. Guerrero, Dr J. Ortiz, Dr J. Ten, Dr R. Bernabeu.
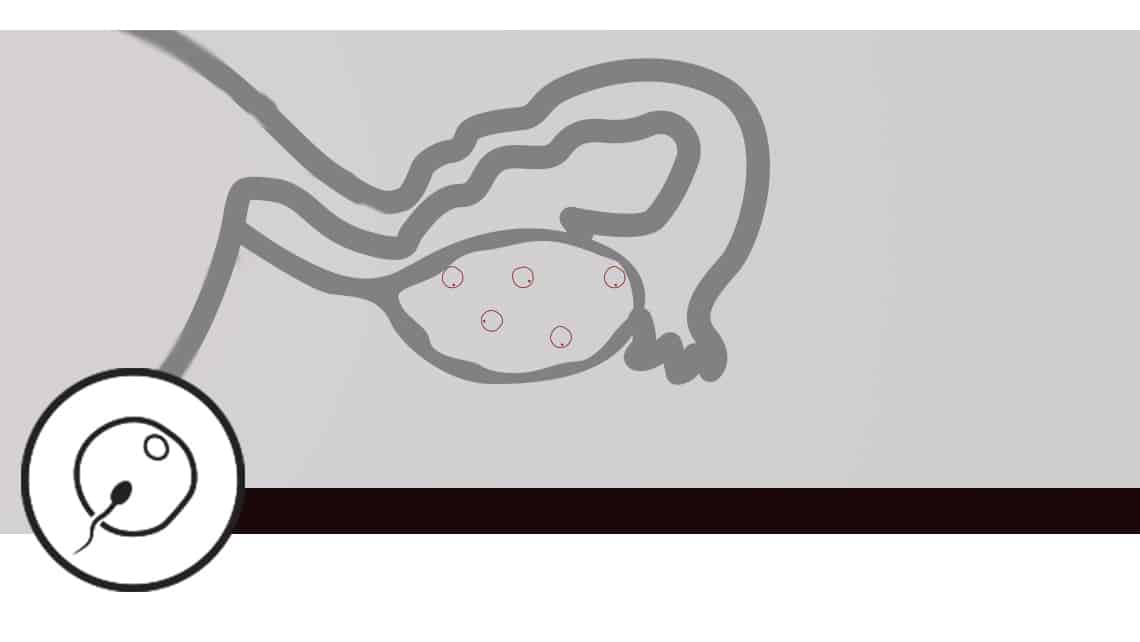
Subluteal study in low ovarian response
Investigation subject: assesses and quantifies the possible differences in follicular response to ovarian stimulation for IVF in patients with low response. It compares the effect of a single drug regimen and dose, with administration starting at two different times of the cycle: follicular phase vs. luteal phase, according to randomisation.
The stimulation protocol is identical in both phases
Objective: this is a randomised clinical trial to compare the follicular response to follicular phase stimulation vs. luteal phase stimulation in suboptimal patients. The main objective is the number of total and mature oocytes (MII) obtained in each type of treatment cycle.
Current situation: study completed.
Location: Instituto Bernabeu Alicante & Instituto Bernabeu Palma de Mallorca.
Coordinated by: Dr Jordi Suñol.
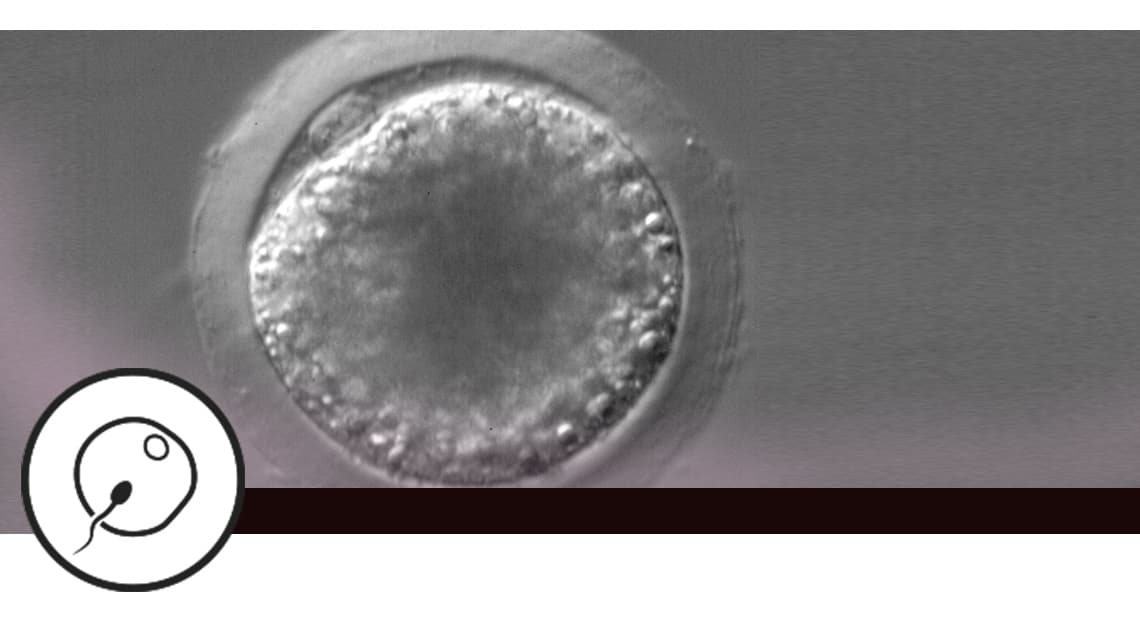
Efficacy of the Random Start protocol in an egg donation programme
Investigation subject: Traditionally, controlled ovarian stimulation strategies have been oriented towards starting in the early follicular phase. However, in recent years a strategy has emerged that has become known as Random-start, aimed at initiating ovarian stimulation regardless of the time of the patient’s cycle. Existing publications on Random-start stimulation include mostly exclusively oncological patients. This strategy may be an alternative to conventional ovarian stimulation in other clinical situations, such as in egg donors.
Objective: To assess the results in terms of clinical gestation and laboratory results in patients undergoing oocyte reception treatment. The eggs come from donors after conventional ovarian stimulation (control) or at different times throughout the menstrual cycle (Random-start protocol).
Current situation: The preliminary results of this work were accepted at the ESHRE 2019 congress and the paper is currently being finalised for publication.
Location: Instituto Bernabeu and Accuna.
Coordinated by: J. Guerrero, Dr J. Castillo, Dr J. Ten, Dr R. Bernabeu.
Investigation subject: prospective, randomised, multicentre clinical trial evaluating the efficacy of two different types of progesterone in cryopreserved embryo transfer cycles.
Objective: to evaluate the clinical gestation rate in patients with vaginal progesterone versus injectable progesterone as luteal phase support.
Current situation: in patient recruitment phase with more than 150 patients recruited.
Location: In Instituto Bernabeu Alicante and Instituto Bernabeu Madrid.
Coordinated by: Dr Cristina García-Ajofrín and Dr Andrea Bernabeu.

Effect of IBgen RIF genetic profiling and adjuvant therapies
Investigation subject: the adjuvant treatment a patient would benefit from according to her genetic profile according to the IBgen RIF test.
Objective: this study aim is to identify IBgen RIF risk genotypes that show a benefit after administration of a given adjuvant treatment.
Current situation: An article is currently being written for publication in a specialised journal in Reproductive Medicine.
Location: Molecular Biology and Genetics Department. Instituto Bernabeu Alicante.
Coordinated by: Dr Belén Lledó and Dr Ana Fabregat.


