Pre-implantation genetic diagnosis or testing (PGD/PGT)
Methods used to detect and avoid transmission to the embryo of serious diseases caused by genetic abnormalities and/or chromosomal abnormalities that sometimes make getting pregnant imposible, cause premature pregnancy loss or lead to the birth o a child with special needs
What is the PGD and why is it important?
Pre-implantation genetic diagnosis (PDG) or the pre-implantation genetic test (PGT) is a genetic analysis of the embryo that is performed by studying a biopsy of its cells prior to transfer to the mother’s uterus. The information obtained means that we can:
- Avoid transfer of embryos that, as a result of chromosomal abnormalities, would lead to premature pregnancy loss or that would not lead to the birth of a healthy child.
- Locate and prevent serious illnesses in the embryo caused by genetic abnormalities, ensure the birth of healthy offspring and end transmission of diseases to future generations.
What are chromosomal and monogenic abnormalities?
Human beings are born with 46 chromosomes. Of these, 23 come from the father and 23 come from the mother. They transmit our inheritance to us. We have 22 autosomes and a couple of sex chromosomes. The latter determine a person’s sex (XX for women and XY for men). Each chromosome is an ‘extensive book’ that contains the bits of genetic information that dictate what each cell in our organism is like and how it functions.
However, errors sometimes occur when cells divide. This can lead to cells with too many copies of a chromosome or, on the contrary, insufficient copies. These abnormalities can be:
- numerical (excess or insufficient chromosomes)
- structural (a chromosome with a missing or excess part; that is inside another chromosome; or that is inverted).
Abnormalities in the number and position of chromosomes in the embryo are the most common cause of implantation failure and premature pregnancy loss, scenarios whose likelihood increases the greater the age of the mother. Therefore, in our unit dedicated to embryo implantation failure, a PGT-A (for aneuploidies) is an incredibly useful tool for diagnosis and treatment.
In other cases, the abnormality is located in a specific gene that affects how an organism functions. This can cause a monogenic disease, or the group of illnesses that can be passed on to offspring.
Types of pre-implantation genetic diagnosis
- Chromosomal abnormality PGT-A. An aneuploidy detection pre-implantation genetic test.
- PGT-M pre-implantation genetic test for detecting monogenic disorders.
- PGT-SR for structural abnormality diagnosis.
These are described in detail below.
1. PGT-A: the aneuploidy analysis pre-implantation genetic test (chromosomal abnormalities)
| Chromosomal abnormalities in embryos are one of the main reasons for low pregnancy rates. Only embryos with a precise quantity of chromosomes lead to the birth of a healthy child. This technique means that abnormal embryos can be ruled out even if, from a physical point of view, they appear to be good quality embryos.
By avoiding transfer, couples can avoid gestating an affected child, embryo implantation failure or premature pregnancy loss. For example, an extra copy in chromosome 21 causes Down’s Syndrome (trisomy 21). Other common chromosomal aneuploidies (an extra or missing chromosome) are trisomy 18, trisomy 15, trisomy 47 and XXY (Klinefelter syndrome). |
Who should have a PGT-A?
- Women who plan to have children when they are older (over 35 years of age)
- Women who have had recurrent pregnancy loss and/or earlier embryo implantation failure in two or more cycles of in vitro fertilisation
- Patients with chromosomal abnormality diagnoses
- Couples in which a severe male factor has been identified as a cause of infertility.
PGT-A testing, step by step
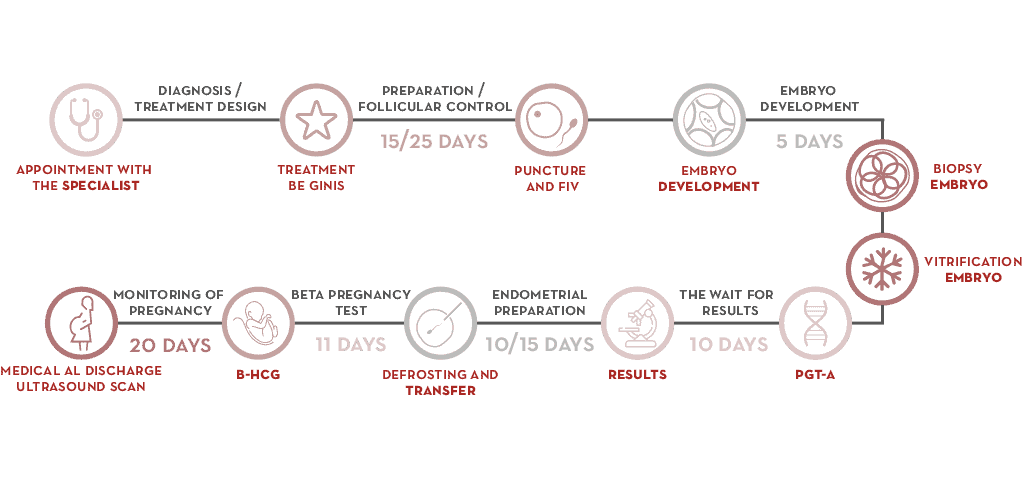
- At the clinic, the gynaecologist designs courses of treatment that begin when the patient’s period starts.
- Ovarian stimulation is performed in order to retrieve oocytes.
- Embryos are retrieved during a cycle of IVF.
- When an embryo is between days 5 and 6 of development (blastocyst stage), several cells are retrieved using the embryo biopsy technique.
- Embryos are cryopreserved until they are transferred.
- The biopsy is processed for chromosome analysis and a diagnosis is reached.
- Preparation of the mother’s endometrium can begin once the result is obtained. Transfer of an embryo with no chromosomal abnormalities is readied and abnormal embryos are ruled out so that unsuccessful transfers can be avoided.
2. PGT-M pre-implantation genetic testing for detecting monogenic disorders
| This consists of a genetic analysis of embryos in couples who are carriers of a hereditary disease. It means that the abnormality or mutation in a gene that causes a disease can be detected.
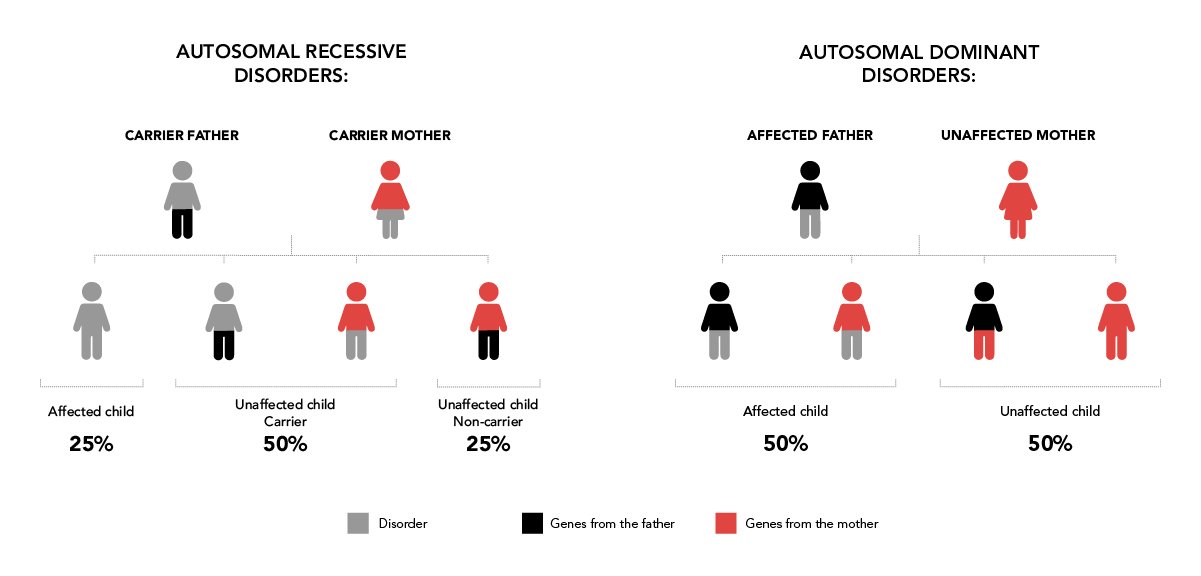
The first step entails performing a genetic analysis on the future parents. The aim is to identify the error in the gene (mutation) that causes the disease (genetic study). Once we have the genetic study, next step would be to undergo an informativity testing which will allow us design a specific diagnosis strategy for the family’s disease. Disorders can be autosomal recessive, autosomal dominant and linked to the X chromosome (for example, fragile X syndrome, haemophilia, cystic fibrosis, Huntington’s disease, sickle cell anaemia, Marfan syndrome and so on). |
Who should have a PGT-M?
- Couples in which one partner has a genetic disorder with dominant inheritance (50% of children are born with the disorder).
- Couples in which the mother is a carrier of a genetic disorder that is linked to the child’s sex (50% of children have the disorder).
- Couples in which both partners are carriers of a genetic disorder with recessive inheritance (25% of children are born with the disorder).
PGT-M step by step
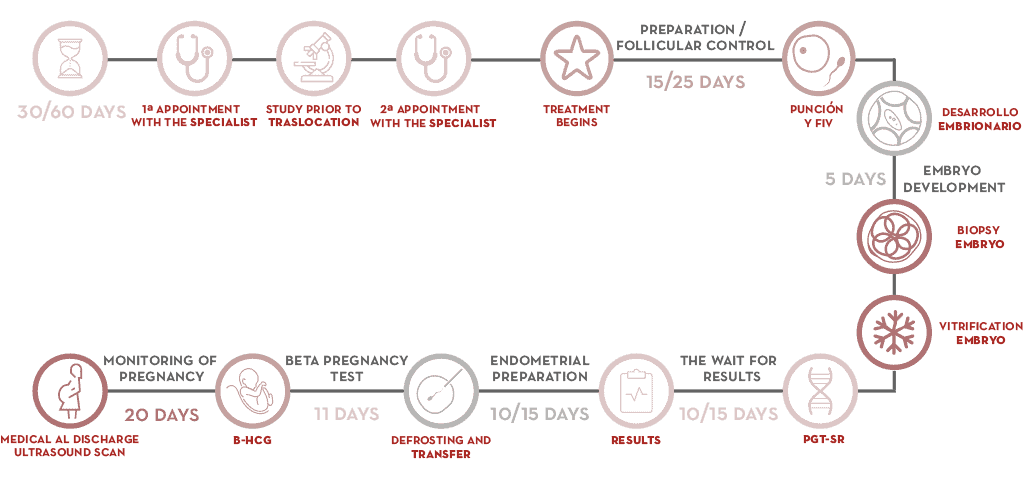
- The first step is a genetic analysis of both parents. Each case must address identification of the error in the gene (mutation) that causes the disease.
- After this, an informativity study is carried out. This consists of developing a strategy that facilitates identification of the abnormality in the embryos. Healthy and affected family members are often required to participate in the study.
- A cycle of in vitro fertilisation treatment can then begin. Once the female patient has her period, a course of controlled ovarian stimulation begins. Between 15 and 25 days later, oocytes are retrieved.
- The ooctyes are fertilised in the laboratory using semen from the woman’s partner or from a donor. They are developed until day 5 or 6 of the embryo (blastocyst stage) and this is when an embryo biopsy is performed. It consists of removing several cells for genetic analysis. The embryos are cryopreserved until the results are obtained.
- The biopsy is processed for genetic analysis and a diagnosis is reached.
- Once the result has been obtained, the mother’s endometrium is prepared and transfer of an embryo without the analysed gene is readied.
3. PGT-SR: the pre-implantation genetic test for detecting structural abnormalities
| This test detects embryos with abnormal chromosomes because they are ‘broken’ or because there are incorrect links between segments. Structural chromosomal abnormalities such as these vary in type. They can include deletions, translocations, duplicates, insertions, inversions and ring formation. A person is affected by the disorder when a gene cannot express itself correctly as a result of the abnormality in the chromosome’s structure. |
Types of structural chromosomal abnormalities
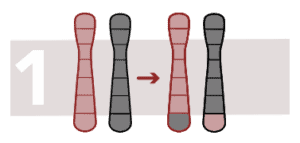
Balanced reciprocal translocation
Translocation is a type of chromosomal abnormality in which the chromosome breaks and one part joins onto another chromosome. It is reciprocal when it occurs due to transfer of segments between two chromosomes and changes to the configuration but not the number of chromosomes.
Balanced reciprocal translocation occurs when the rearrangement does not generate a loss off or excess chromosome material. It occurs when a chromosome region changes position in the genome.
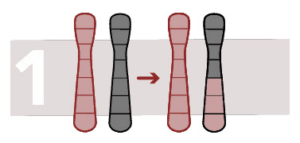
Unbalanced reciprocal translocation
This rearrangement causes a loss or addition of chromosome material. There is a change in the number of copies in a chromosome region. Fragments of a chromosome can be found in another chromosome.
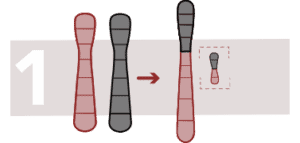
Robertsonian translocation
This generates a fusion of two acrocentric chromosomes (with a single arm), the two extremes are lost and the two chromosomes fuse. Sufferers have 45 chromosomes instead of 46 and there is an increased risk of trisomy.
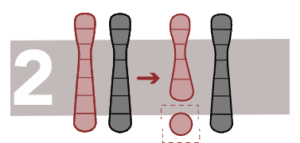
Deletions
This is the loss of a fragment of DNA in a chromosome.
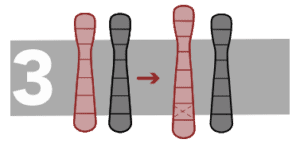
Duplicates
A chromosome segment is repeated after the original fragment and it produces one or more copies of a gene or a chromosome region.
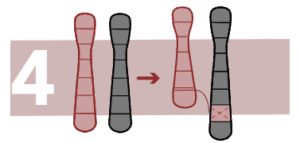
Insertions
A part of a chromosome inserts in an unusual position inside the chromosome itself or inside another chromosome. If there is no added or lost chromosomal material, the person will not be affected.
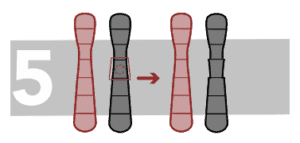
Paracentric inversion
Inversion occurs when a part of a chromosome breaks in two points and the inner segment turns inside out and re-joins. Paracentric inversions happen when the inversion does not involve the centromere.
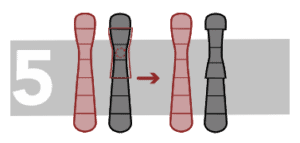
Pericentric inversion
In this case, the inverted fragment includes the centromere.
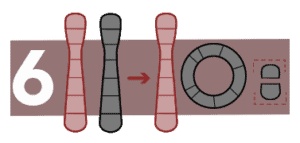
Ring formation
In this case, the ends of a chromosome break away and fuse to form a ring. It causes genetic disorders, the most frequent of which is Turner syndrome.
Who are the tests suitable for?
Use of these tests is recommended when one partner is a carrier of a structural chromosomal abnormality.
PGT-SR step by step
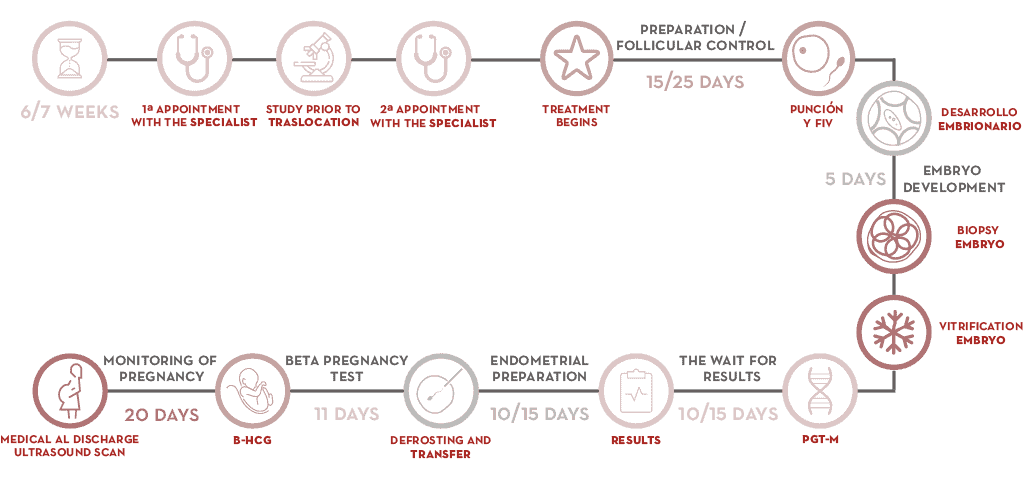
- Initial consultations entail assessing the patients’ case.
- A previous structure alteration study might be required.
- At the next appointment, the gynaecologist designs a course of treatment that begins when the patient’s period starts.
- Ovarian stimulation is performed in order to retrieve oocytes.
- Embryos are retrieved during a cycle of IVF.
- When an embryo is between days 5 and 6 of development (blastocyst stage), several cells are retrieved using the embryo biopsy technique.
- Embryos are cryopreserved until they are transferred.
- The biopsy is processed for chromosome analysis and a diagnosis is reached.
- When the result has been obtained, the mother’s endometrial preparation can begin. Transfer of an embryo with no chromosomal abnormalities is readied. Abnormal embryos are ruled out and unsuccessful transfers are avoided.
The advantages of undergoing PGD/PGT
PGD/PGT improves embryo selection. We know which embryos do not have an abnormal number of chromosomes and will lead to the birth of a healthy child.
Transfer of embryos that will not implant can be avoided. Certain chromosomal abnormalities are incompatible with life and prevent embryos from developing during the early stages or from implanting in the mother’s uterus.
Transfer of embryos that will lead to pregnancy loss or the birth of children with a variety of syndromes can be avoided.
The time taken to get pregnant is reduced. Embryos that will not lead to the birth of a healthy child or that will block as they develop are not transferred.
The financial burden is decreased. The process removes the need to freeze and transfer embryos that are not genetically healthy, even if they might appear to be healthy. This removes the cost of transferring embryos that we know will not develop.
A positive impact on psychological wellbeing. There is a guarantee that the embryo is healthy and a decreased risk of pregnancy loss. This eases the emotional stress experienced by couples.
The disadvantages of PGD/PGT
It is an invasive procedure because a biopsy has to be performed on the embryo so that genetic tests can be carried out.
A cycle with no transfer. There is a risk of all the embryos being chromosomally abnormal, meaning that transfer cannot take place. This means that treatment must stop and this has a significant emotional impact on patients.
Embryo mosaicism. Thanks to the precision of genetic analysis techniques, we are able to determine if an embryo has normal cells or abnormal cells known as mosaicism. It is yet to be determined if this affects the embryo in any way. A number of research projects performed by Instituto Bernabeu are looking into this.
Screening method. Embryo biopsies analyse only external sections and leave sections that gives rise to the baby intact. Several scientific research projects have demonstrated an elevated correlation between the two. We accept that the biopsy sample that is taken is representative of the entire embryo.
A difficult decision to have to take. Many couples find analysing their embryos a difficult decision to have to take. In addition, at our clinic, patients are given genetic, psychological and professional advisory services to guide them in the process if they wish.